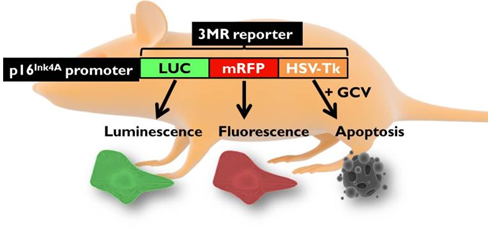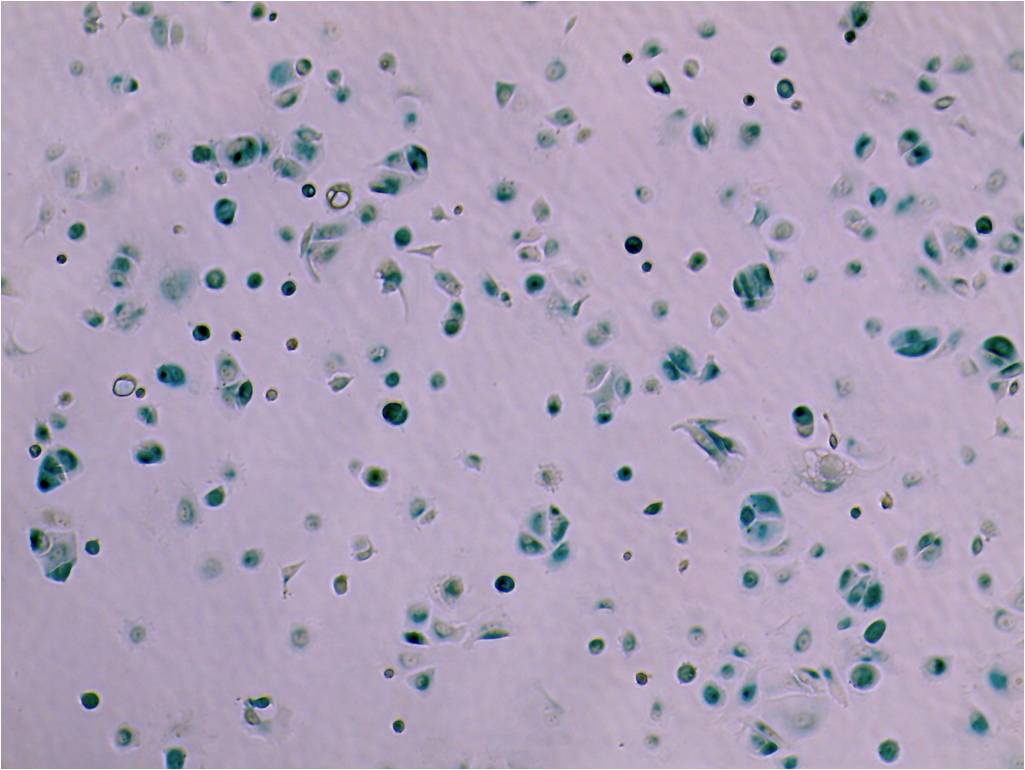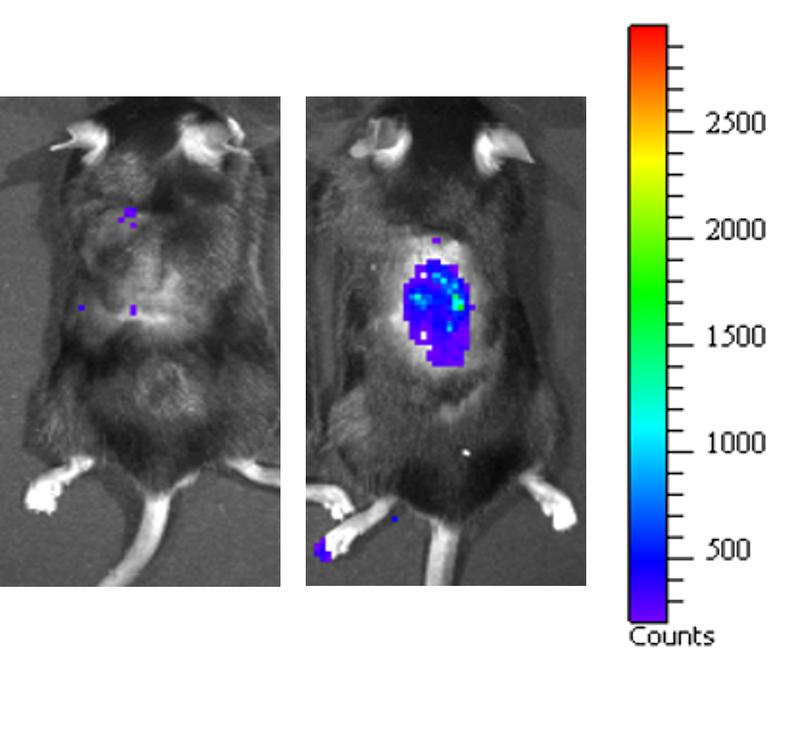
Aging is a progressive and generalized deterioration of the functional capacities of an organism. One common feature of older organisms is the accumulation of cellular senescence (Borghesan et al., 2020). Senescent cells are characterized by the engagement of the cyclin-dependent kinase (CDK) inhibitors p21Cip1 and p16Ink4a, which arrest cell cycle progression at the G1-S phase (Gorgoulis et al., 2019), together with a number of morphological alterations and the development of a peculiar and heterogeneous secretory phenotype, called Senescence-Associated Secretory Phenotype (SASP) (Hernandez-Segura et al., 2018). By using a newly developed mouse model for the detection and elimination of senescent cells in vivo—the p16-3MR model—we have demonstrated that senescent cells accelerate and optimize cutaneous wound healing through the SASP (Demaria et al., 2014). In contrast, senescent cells accumulate with aging and at sites of age-related pathologies, while the persistent secretion of the pro-inflammatory SASP can alter tissue microenvironments generating low levels of chronic inflammation, contributing to several major diseases, including cancer (Borghesan et al., 2020; Wang et al., 2020).
Our lab is trying to answer a number of fundamental questions: how many senescence programs exist in vivo and can we distinguish beneficial and detrimental senescent cells? What are the cell-extrinsic and –intrinsic mechanisms determining the accumulation and persistence of senescent cells? What are the main SASP factors that promote disease? What are the best therapeutic strategies to limit the detrimental functions of senescent cells?
|
Research Lines
Molecular characterization of the senescence heterogeneityWe have recently identified the gene expression profile of different senescence-associated programs that are dictated by the senescence inducer, the cell type and the time spent by the cell in a senescence state (Hernandez-Segura et al., 2017). We have also shown the importance of the microenvironment for the development of senescence-associated features, in particular the role of oxygen-responsive pathways (Van Vliet et al., 2021; Casciaro et al., 2021). However, even within a predicted homogenous population of senescent cells (where all cells are of the same type and treated with the same stress at the same time) we observe cell-to-cell variability in the expression of senescence markers. We are now interested in deconvolving the intra-population heterogeneity by adopting single-cell qPCR and RNAseq technologies using cell culture and in vivo systems.
Induction, accumulation and persistence of age-related senescent cellsDuring the past few years, we have demonstrated that senescent cells persist in tissues during aging, while they are efficiently removed in wound healing (Demaria et al., 2017; Demaria et al., 2014). The detailed mechanisms that determine whether senescent cells are eliminated remain largely unknown. To better understand such mechanisms, and how they might be altered during aging, we are focusing on three aspects: 1) stresses that induce senescent cells in vivo; 2) activation of anti-apoptotic pathways as part of the senescence response; 3) cell-extrinsic and cell-intrinsic mechanisms that determine immune clearance of senescent cells.
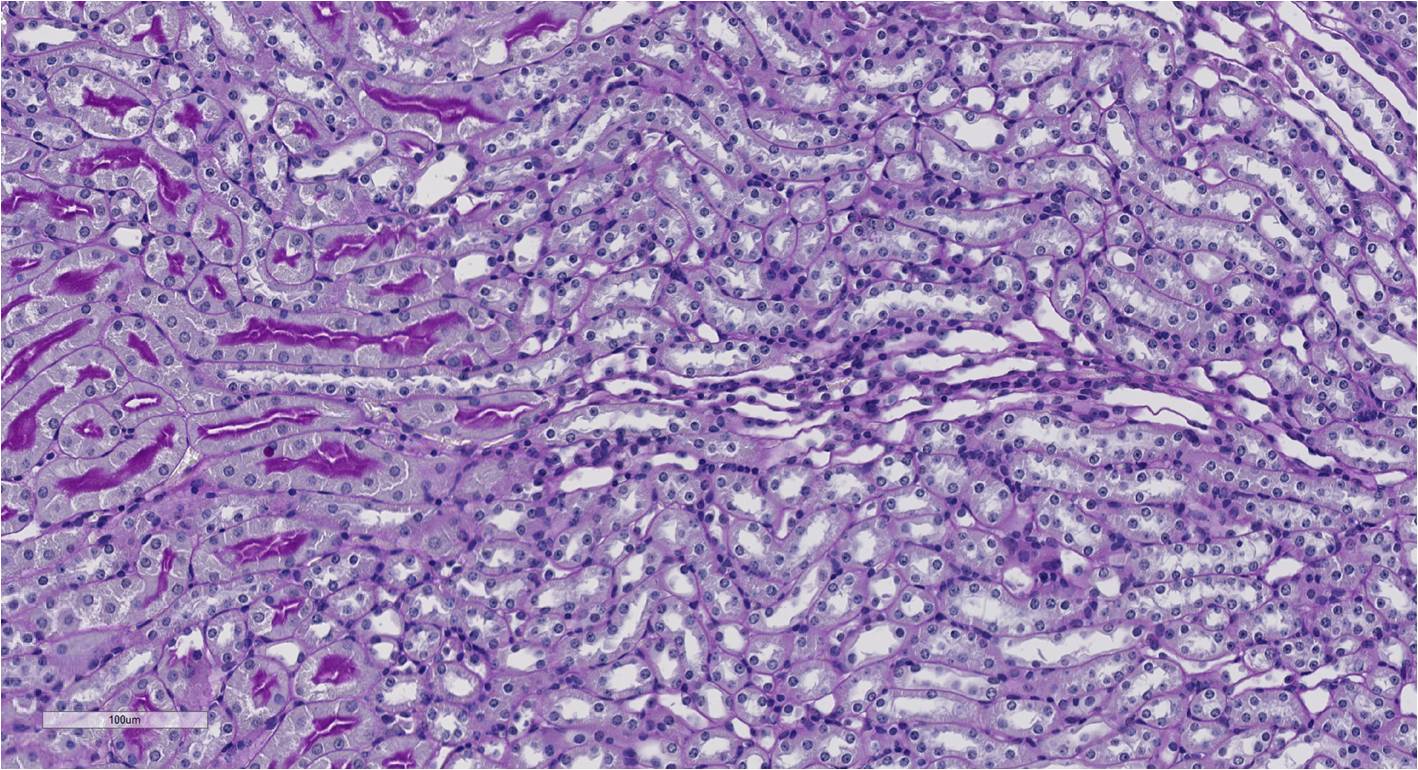
Senescence-associated mechanisms determining pro-disease functionsWe have previously shown that senescent cells contribute to a number of age-related diseases, such as cancer, osteoarthritis and neurodegeneration (Chang et al., 2016; Chinta et al., 2018; Demaria et al., 2017; Jeon et al., 2017). However, how senescent cells drive the onset and progression of such diseases is not yet understood. To study the specific mechanisms by which senescent cells promote diseases, we are characterizing the gene expression profile of disease-associated senescent cells using a number of –omics techniques. Currently, we are focusing on studying senescent cells isolated from naturally aging or cancer-bearing mice. Our initial effort is to identify specific SASP components that are secreted by senescent cells and that can contribute to disease.
Lifestyle effects on induction of cellular senescenceWe have recently shown that accumulation of senescent cells can be prevented or delayed by reducing calories intake in both mice and humans (Fontana et al., 2018), and we have preliminary data showing that endurance exercise in humans also slows senescence accumulation (unpublished). Now, we are planning to expand the analysis to additional lifestyle/environmental conditions. We are currently studying the contribution of diverse dietary regimens (high/low fat, high/low protein, high/low carbs), mild or intense exercise, and exposure to UV radiation for the accumulation and persistence of senescent cells in mice.
Biomarkers and identification of senescenceUsing data collected from our studies, we are compiling lists of biomarkers that can define and specify senescence in culture and in vitro. We have recently designed a step-by-step protocol to validate the presence of senescent cells (Kohli et al., 2021).
Development of senotherapiesIn parallel to the understanding of the mechanisms regulating senescence-associated phenotypes, we are interested to develop senotherapies based on pharmaceutical, nutraceutical or lifestyle interventions. In order to facilitate the development of pharmaceutical approaches, our lab also partners with Cleara Biotech, a company devoted to designing and testing novel senolytic approaches for the treatment of a variety of age-related dysfunctions.
Senescence in humansThanks to a number of collaboration with clinical departments, we are monitoring conditions in which senescent cells accumulate in humans. We are interested in natural accumulation with aging, premature induction due to drug treatments and association to age-related pathologies.
Our collaborations We have formed collaborations with many leading scientists in the fields of aging and senescence: Dr Judith Campisi (Buck Institute, USA) -> heterogeneity of senescence Dr Rob Coppes (UMC Groningen, NL) -> effect of removing senescent cells for salivary gland regeneration Dr Peter de Keizer (UMC Utrecht, NL) -> development of novel senolytics Dr Luigi Fontana (Univ Sidney, AU) -> impact of calorie restriction on accumulation of senescent cells Dr Jourik Gietema (UMC Groningen, NL) -> induction of premature senescence in testicular cancer survivors Dr. Vassilis Gorgoulis (University of Athens, Greece) -> Identification and characterization of senescent cells in premalignant lesions Dr Agnes Jager (UMC Rotterdam, NL) -> induction and phenotype of senescent cells in breast cancer patients Dr Wim Quax (Univ of Groningen, NL) -> activation of apoptosis-related mechanisms during senescence Dr. John Speakman, (University of Aberdeen/Chinese Academy of Sciences) -> modulation of aging phenotypes via dietary interventions Follow us on social media
|